Does every atom except hydrogen have an octet. What is the hybridization on the central atom.
Drawing Dot Structures Video Khan Academy
It is also the atom having low electronegativity.
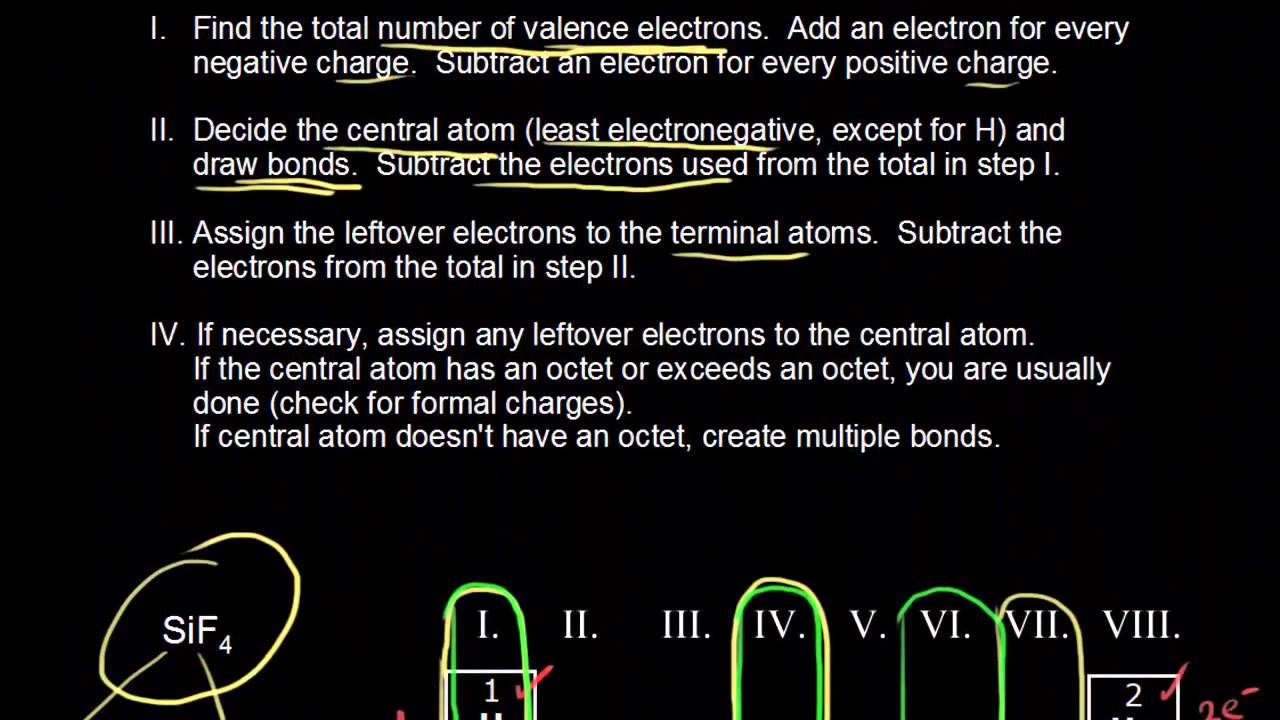
. Hereof how do you find the central atom when drawing a Lewis structure. We have found the total number of valence electrons. Decide which is the central atom in the structure.
If it is the latter sodium then its because this is an ionic compound and we build the lewis structures of the cation and anion separately. The order of electronegativity for the nonmetals is FONClBrISCH. The central atom in a Lewis structure is usually the least electronegative atom.
Experts are tested by Chegg as specialists in their subject area. Eight electrons come from one double bond pair of B-Cl and two single bond pairs of B-Cl on the boron central atom of BCl3. Simple molecules CFH NH HS EG.
Rules for Drawing Lewis Structure. A If there are more than one of the least electronegative atom your skeletal structure should have those two attached to one another EXCEPTION. The central atom AS has 4 electron groups around it.
When drawing the Lewis structure the central atom is generally the. Decide on a skeletal structure What is bonded to what The central atom is generally written first in the formula Hydrogen is never the central atom even if it is. Write the Lewis structure for CH2O where carbon is the central atom.
B Draw a Lewis structure for IO in which the octet rule is. Usually the central atom will be the one that has the most unpaired valence electrons. Determine the total number of electrons available for bonding.
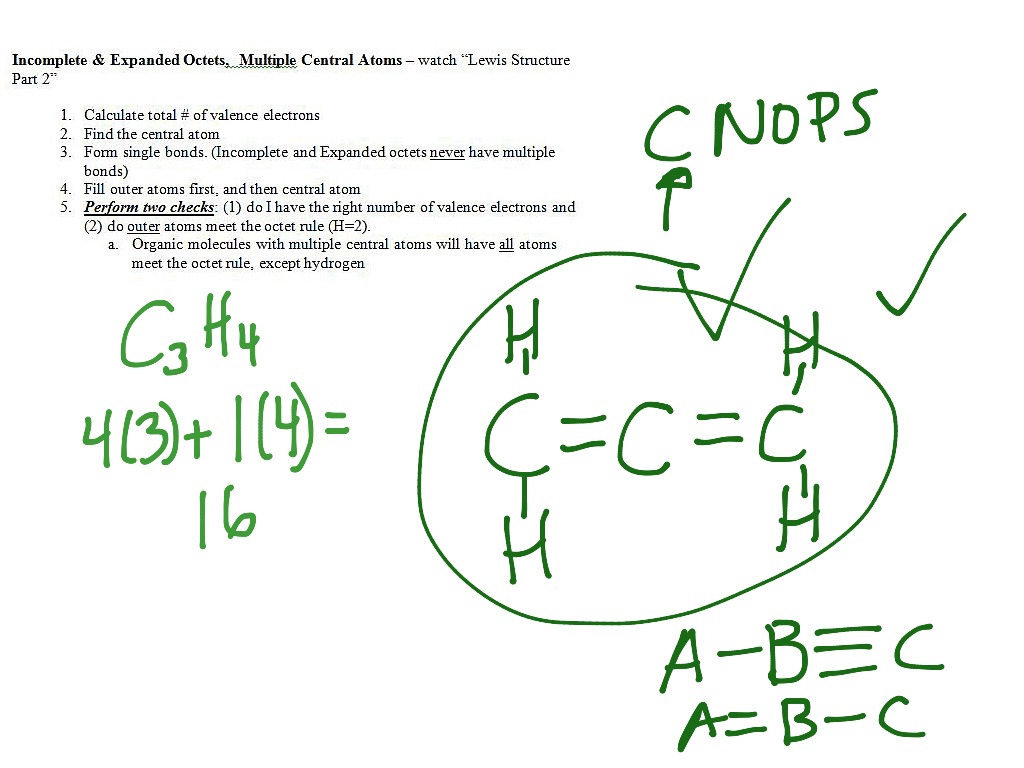
The Lewis structure for Asis has 3 bonding pairs. Include all lone pairs. VIDEO Finding the Central Atom of an Electron Dot Structure Lewis Structure Examples.
The core atom in the BCl3 Lewis structure is boron which is bonded to the three chlorine atoms by single bonds B-Cl. Hydrogen is never the central atom. Determine the total number of valence electrons to be depicted in the Lewis diagram.
Here is an Electronegativity Table. The central atom is also usually the least electronegative. Metals will almost always be the central atom if they are present.
See answer 1 Best Answer. Draw a skeleton structure in which the other atoms are single-bonded to the central atom. Determine how many electrons must be added to central element.
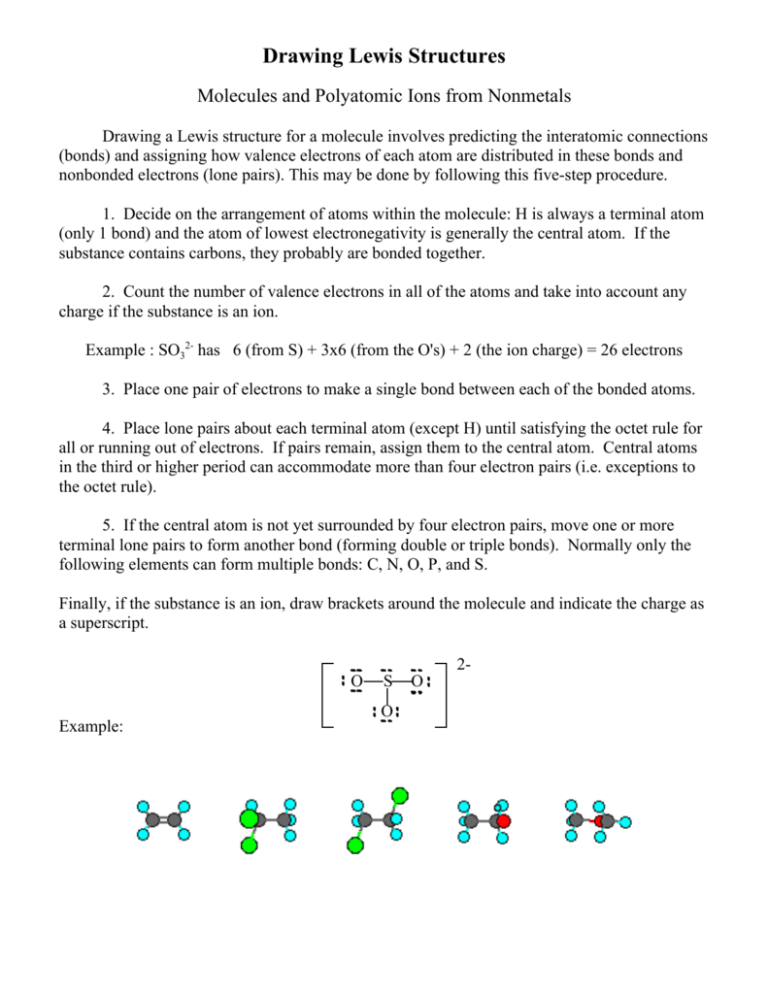
MUST FOLLOW IN ORDER. The atom with the least electronegative charge should. In drawing Lewis structures for relatively small molecules and polyatomic ions the structures tend to be more stable when they are compact and symmetrical rather than extended chains of atoms.
A Lewis diagram is a representation of the valence shell electrons in a molecule where each atom participating in the bond tries to acquire the octet structure. If an atom occurs only once this atom is. Usually the central atom will be the one that has the most unpaired valence electrons.
CO 2 Total 16. Once we know how many valence electrons there are in PH3 we can distribute them around the central atom and attempt to fill the outer shells of each atom. A The Lewis structure for AsCly has a total of 10 lone pairs.
With the help of three single bonds it already shares six electrons. Now as we continue to review how to draw Lewis structures we must decide which atom will be drawn in the center of our Lewis structure. The principle is based on taking the total number of valence electrons of the atoms in the molecule and redistribute them between all.
The Lewis structure for PH3 is similar the the structure for NH3 since both P and N. How to Draw a Lewis Dot Structure Step 1. Remember that hydrogen H only needs two valence electrons to have a full outershell.
Find the central atom in the e lectron d ot s tructure Lewis Structure using the method you find best. What this means is that we decide how the atoms are to be bonded. Here are the steps I follow when drawing a Lewis structure.
Choose a central atom well start with small molecule examples for which there is only one central atom and the other atoms - the peripheral atoms -. A Draw a Lewis diagram for IO4 in which the central I atom has a formal charge of zero and show all NONZERO formal charges on all atoms. What is the molecular geometry around the central atom.
Can hydrogen be a central atom for. The PH3 Lewis structure has 8 valence electrons. Draw a skeletal structure.
Dsp3 sp sp 3 sp Submit Show Hints. Arrange the atoms as they will be bonded together. Draw the Lewis structures for the following molecules.
First and foremost carbon EN25 is less electronegative than nitrogen EN31 so by your rule it should be in the center. Molecules with multiple bonds O2 СО CHO EG. We review their content and use your feedback to keep the quality high.
Draw the Lewis structure for KrF4 and answer the following question. The central atom is also usually the least electronegative. To find electronegativity either rely on periodic table trends or consult a table that lists electronegativity values.
That will normally be the least electronegative atom Se. Draw the Lewis structure for HCN where is the central atom. If all of the atoms usually form the same number of bonds the least electronegative atom is usually the central atom.
The central atom in a Lewis structure is usually the least electronegative atomEDITIf an atom occurs only once this atom is most likely to be the central atom exception is hydrogen as it can. The central atom of a molecule is usually the least electronegative atom or the atom with the highest valence. Draw a trial structure by putting electron pairs around every atom until each gets an octet.
In the IO4 ion the I atom is the central atom. Place least electronegative element in center and draw single bonds from the central atom to other atoms. The central atom is usually the atom with the lowest subscript in the molecular formula and the atom that can form the most bonds.
Count the total number of valence electrons in the structure Remember Group of Valence electrons 2. Calculate the total number of valence electrons in the molecule by adding the valence electrons from each atom. What is the electron geometry around the central atom.

Lewis Structures Multiple Central Atoms Science Chemistry Chemical Bonds Showme

Step 1 Identify The Central Atom And Draw It S Lewis Structure This Is The Element With The Lowest Number Of Atoms In The Formula Draw A Lewis Dot Diagram Ppt Download

Rules For Lewis Structures Pre Ap Adapted From Brown And Lemay Ppt Download
Rules For Drawing Lewis Structures

How To Draw Lewis Structures For A Molecule With One Central Atom No Octet Rule Exceptions Chemistry Study Com

Ppt Drawing Lewis Structures Powerpoint Presentation Free Download Id 529421


0 comments
Post a Comment